So What Happened to the Pfizer Vaccine Trial Participants?
Revisiting the Vioxx $4.85 billion Settlement

StoptheShots is trending again on twitter, as it has most days for the past several months. Of course the jabs are still being called safe and effective just like the facemasks they need to bring back. Uptake of the Covid “vaccines” seems to have dropped precipitously, which is good, but why are these things even still on the market? Pfizer and Moderna are going to investigate themselves over the myocarditis risks in young people from their mandated products. Some people still cannot work or travel without an updated booster. The emperor has no clothes.
What does it take to really stop the shots and prosecute those responsible? I think we have to go back to the trial data. If the rate of serious adverse events was 1 in 800 in the Pfizer trial, then they would know that their product would cause material harm to millions of people once it was rolled out to so much of the world’s population. Pfizer almost certainly intentionally kept sample sizes very low, threw people out of the analysis, and of course unblinded the study two years before it was over, eliminating the placebo group.
A single death or serious adverse reaction in a clinical trial would need to be reported and extrapolated out to the entire target population this intervention is meant for. That is done to look for rare signals, but the damages can be calculated out. In the Pfizer children’s arm, for example, one 12 year old girl, Maddie de Garay, suffered disability and became wheelchair bound. Pfizer classified her injury as abdominal pain, because otherwise the rate of the jab causing permanent disability would have to be set to 1 in 1200 (the size of the study arm), which is insane for an illness posing statistically no risk to the age group. Pfizer would do anything to hide the bodies of those who might have died or been severely long term injured from the jabs. I speculated on that myself regarding a missing VAERS report many months ago:
All of this had me musing about the drug settlement for Merck’s painkiller Vioxx causing heart attacks. The $4.85 billion was the biggest at the time it happened. It was unwound due to the trial data. Here’s excerpts from this timeline:
At the second meeting of the VIGOR safety panel, the discussion focuses on heart problems. As of Nov. 1, 1999, 79 patients out of 4,000 taking Vioxx have had serious heart problems or have died, compared with 41 patients taking naproxen. The minutes of the panel's November meeting note that "while the trends are disconcerting, the numbers of events are small." The panel votes to continue the study and to meet again in a month.
May 2000: Merck submits VIGOR paper to the New England Journal of Medicine (NEJM) for publication. The data include only 17 of the 20 heart attacks Vioxx patients have.
July 5, 2000: A memo from Merck statistician Deborah Shapiro to Merck scientist Alise Reicin (both are listed as authors of the NEJM paper) refers to heart attacks 18, 19 and 20 suffered by patients taking Vioxx during the study.
July 2000/November 2000: VIGOR authors submit two sets of corrections to their NEJM manuscript. No mention of the three additional heart attacks.
Oct. 13, 2000: Merck tells the FDA about heart attacks 18, 19 and 20.
Nov. 23, 2000: The VIGOR results are published in NEJM, still with no mention of the three additional heart attacks in the Vioxx group. The published results also leave out data on many other kinds of cardiovascular adverse events.
September 2004: Merck withdraws Vioxx after a colon-polyp prevention study, called APPROVe, shows that the drug raises the risk of heart attacks after 18 months. By the time Vioxx is withdrawn from market, an estimated 20 million Americans have taken the drug.
Research later published in the medical journal Lancet estimates that 88,000 Americans had heart attacks from taking Vioxx, and 38,000 of them died.
July 14, 2005: NEJM editor-in-chief Dr. Jeffrey Drazen tells NPR that the journal had been "hoodwinked" by Merck, and that the authors of the VIGOR paper should have told the journal about the additional data.
August 2005: A Texas state jury returns a verdict against Merck in the first Vioxx liability case to go to trial. Some 13,000 lawsuits have been filed against the company on behalf of 23,000 plaintiffs who allege the drug caused heart attacks and strokes.
November 2007: Merck announces it will pay $4.85 billion to end thousands of lawsuits over its painkiller Vioxx. The amount, to be paid into a so-called settlement fund, is believed to be the largest drug settlement ever.
The Whitehouse Station, N.J.-based drug maker emphasized that it is not admitting fault.
The settlement lets Merck avoid the personal-injury lawsuits of some 47,000 plaintiffs, and about 265 potential class-action cases filed by people or family members who claimed the drug proved fatal or injured its users.
So the drug was pulled and taken off the market due to the results from one of Merck’s early trials showing an increased risk of heart attacks. It seems that Merck also tried to hide some of the adverse events in the trial (and what happened to the almost double total mortality in the drug arm?), throwing people out of the analysis for not dying before their study cutoff date, which they made one month earlier than planned to intentionally hide three bodies (victim 18, 19 and 20).
All of this is extremely familiar. Big pharma has just gotten a lot more brazen about it and have gone for psyops instead for the big payday. There’s no need to complete a trial when we are the trial.
The thing about this though is not everybody died of a heart attack who took Vioxx. If the estimate of 88,000 heart attacks after 20 million people had taken the drug is to be believed, then that works out to a heart attack rate caused by the drug in one out of 227 people. This actually lines up well with Merck’s own VIGOR drug study, where 20 heart attacks were investigated in a drug arm of 4000, equaling a death rate of one in 200.
It still would equal millions permanently injured for a gene therapy intended to be rolled out to the entire world population, and the suspicious higher mortality in the vaccine arm was never investigated. Of course vaccines under Emergency Use Authorization have a liability shield, so don’t sue Pfizer just take it up with the government. I’m sure that’s working out great.
It seems to me that the liability shield would go away if Pfizer engaged in willful fraud. Even under an EUA, which was probably put in place so that the jab makers could get their juice in the arms of everyone on Earth.
What I’m really wondering is what happened to the original trial participants. It’s been more than two years for most of them since their first jab, and I have to assume and hope that a significant portion of them are still alive and well. But is anybody following up on them? With the all cause mortality remaining elevated even now, it is possible that these jabs have slow kill effects, which could only be learned about from the study arm that was supposed to be monitored for two years, but which was unblinded and mostly took the real jabs already. And what became of the superheroes in the Pfizer kiddie study? Are they okay?
Sometimes one person matters…I’d love to know the thoughts of trial participants now…




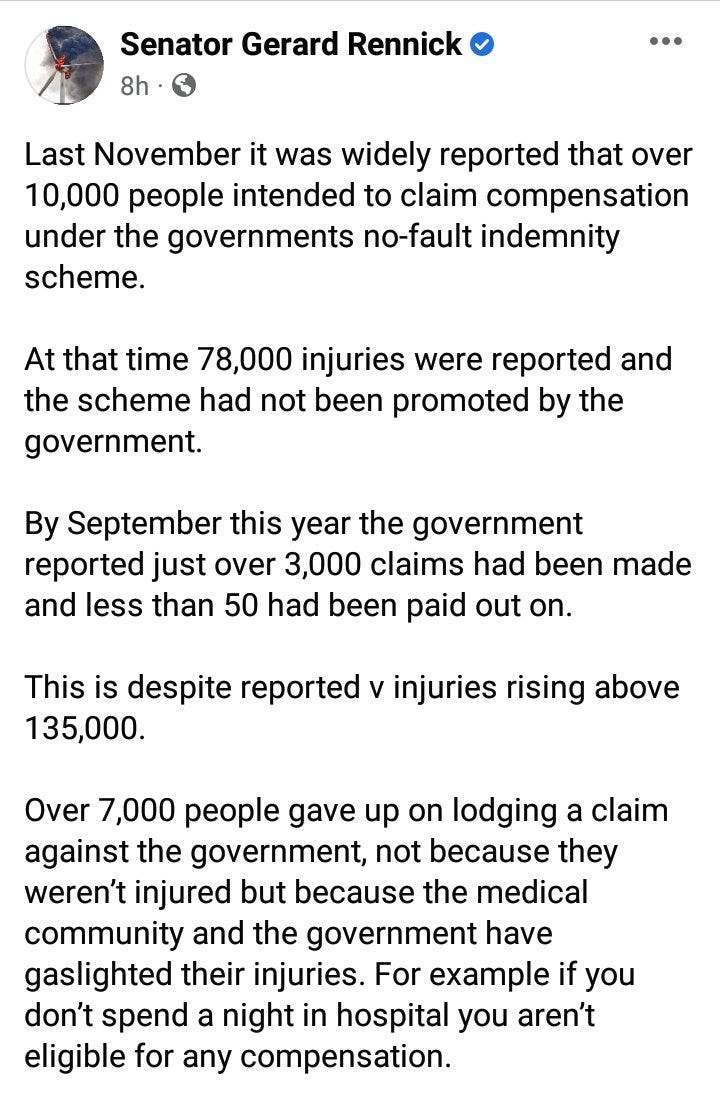

Over 10K enrollees were "excluded" (and never followed up on) from the trials AFTER they'd received one or more jabs, and their data never appeared in the final report. It was at this point these people were no longer considered "participants".
In the final report, they placed a red herring statement that said "Of the participants" only a tiny % had been excluded. The final report did NOT mention the over 10K people who WERE jabbed and dropped out of the trials. Once they moved a person over to the earlier exclusion list, they were no longer considered to be "participants" even though they HAD been jabbed. It was just a word salad game.
But I found the earlier exclusion lists HIDDEN amongst 1,000s of documents in their "find the banana in the picture game" when they were forced to release the data. The links are in my story below.
SEE: https://ghostofjfk.substack.com/p/pfizer-data
Merck's lead attorney on the Vioxx case, Kenneth C. Frazier, was promoted to CEO of Merck after the settlement -- because he did such a good job of keeping the number low. Then, as CEO, Frazier pushed Merck's HPV vaccine -- and many people joked that "HPV" stands for Help Pay for Vioxx (because having this new billion dollar vaccine helped saved the company from bankruptcy). Frazier retired in 2021 but continues as the Executive Chairman of Merck’s board of directors. That's how f'ed up everything is.